Abstract
Background: HLH is a systemic condition characterized by inflammatory storm and immune-mediated organ damage. Adult M-HLH is particularly challenging with median overall survival (OS) <2 mos in most retrospective analyses due to aggressive presentation and HLH features often obscured by manifestations of the underlying malignancy or its directed therapy.
Methods: We prospectively analyzed adult (≥18 yrs) pts diagnosed with M-HLH between January 2014 and September 2017 at MD Anderson Cancer Center who fulfilled ≥5 HLH-2004 criteria.
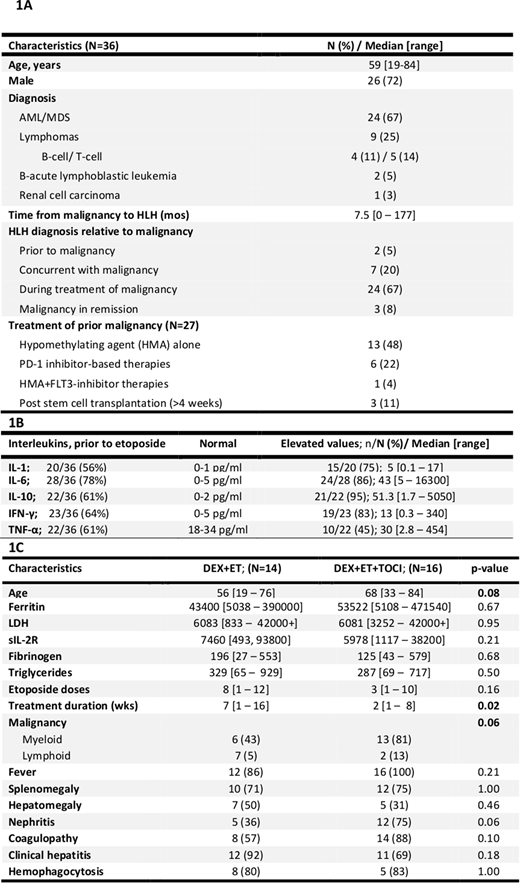
Results: 36 pts with median (med) age 59 yrs (range 19-84) were enrolled (Fig 1A). Med ferritin at diagnosis was 58800 mg/L range 3059-471540); 35 (97%), 30 (83%), 26 (72%), and 18 (50%) pts had ferritin levels >5000, >10000, >25000, and >50000 mg/L, respectively. 23/32 (72%) pts had high sIL2R (med 7010; range 749-127000). Tissue hemophagocytosis was seen in 18 (50%) pts. Other features at diagnosis: fever (n=33; 92%), splenomegaly (n=26; 72%), new ≥ 2 cytopenias (n=26; 72%), triglycerides ≥265 mg/dL (n=23; 64%), fibrinogen <150 mg/dL (n=16; 44%), and low-absent NK cell activity (12/12 tested pts). In addition to sIL2R, baseline cytokine levels done at MDACC are reported in fig 1B. Satellite findings (not part of HLH-2004 criteria but of importance in HLH based on published literature): LDH>5xULN at diagnosis in 33 pts (92%) with med of 6420 (range 833-42000+); 16 (47%) pts at LDH ≥10000. Clinical hepatitis, coagulopathy and nephritis were seen in 28 (78%), 25 (69%) and 20 (56%) pts, respectively. 27 (75%) pts had central nervous systems symptoms; 12/18 (67%) evaluated pts had lymphocytic pleocytosis suspicious for CNS HLH on lumbar punctures. Potential secondary infectious triggers were identified in 16 pts (44%): 5 pts were EBV positive by PCR in blood and bone marrow, 4 had positive CMV PCR in blood, and 7 were found to have various disseminated fungal infections. 17 (47%) pts were tested for hypomorphic mutations and 4 (24%), all male with med age of 55 yrs (range 33-59), tested positive for known HLH mutations including STXBP2 (n=2), BIRC4 (n=1), and PRF1 genes (n=1).
34 (94%) pts received HLH-directed therapy for a med of 2.3 weeks (range 1-16): 4 (11%) received dexamethasone (D) alone at 10 mg/m2 BID (med 1 week; range 1-2); 14 (39%) received D+etoposide (E) with med of 4 (range 1-12) E doses, and 16 (44%) received D+E+tocilizumab (T) with med of 4 (range 1-10) E doses and med of 1.5 (range 1-4) T doses (Fig 1C); 2 (6%) pts declined therapy. 14 (39%) pts received concomitant systemic therapy for their underlying malignancy including 6 with D+E, 7 with D+E+T, and 1 with D only . Pts received broad-spectrum antimicrobials, intrathecal chemotherapy, renal replacement therapy and polyvalent immunoglobulins (when indicated).
Overall, 11/34 (32%) pts responded: 5/14 (36%) in D+E group (all had complete response (CR) of whom 1 received allogeneic stem cell transplant (ASCT)) and 6/16 (38%) in D+E+T group (2 CR; 4 partial response (PR)). At med follow-up of 19 mos, med OS of the cohort was 1.6 mo (95% CI: 0.2-23.9), with 1-yr OS of 20%. The 30- and 60-day mortality was 44% and 64%. Med OS for the 11 responders was 6.2 mos vs 0.6 mos in non-responders (p=0.001).
Conclusion: M-HLH has poor outcomes with med OS <2 mos in spite therapy. Etoposide-based therapies with or without malignancy directed therapy induced a response in approximately 30% of pts. Achieving a response to therapy was associated with improved OS. The presence of an active underlying malignancy, advanced age, and poor functional status often precluded ASCT in this population, likely contributing to dismal OS. M-HLH pts are in urgent need of more effective forms of therapy that can be administered concomitantly with cancer directed therapies. Early transition to ASCT may further improve outcomes in adult M-HLH
Kadia:Jazz: Consultancy, Research Funding; BMS: Research Funding; Novartis: Consultancy; Takeda: Consultancy; Takeda: Consultancy; Abbvie: Consultancy; Celgene: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Abbvie: Consultancy; BMS: Research Funding; Jazz: Consultancy, Research Funding; Celgene: Research Funding. Ravandi:Macrogenix: Honoraria, Research Funding; Abbvie: Research Funding; Orsenix: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Jazz: Honoraria; Seattle Genetics: Research Funding; Sunesis: Honoraria; Macrogenix: Honoraria, Research Funding; Abbvie: Research Funding; Seattle Genetics: Research Funding; Xencor: Research Funding; Xencor: Research Funding; Bristol-Myers Squibb: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Bristol-Myers Squibb: Research Funding; Sunesis: Honoraria; Jazz: Honoraria. Jabbour:Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; Takeda: Consultancy, Research Funding; Abbvie: Research Funding. Konopleva:Stemline Therapeutics: Research Funding. Andreeff:Amgen: Consultancy, Research Funding; United Therapeutics: Patents & Royalties: GD2 inhibition in breast cancer ; SentiBio: Equity Ownership; Astra Zeneca: Research Funding; Oncolyze: Equity Ownership; Reata: Equity Ownership; Eutropics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Reata: Equity Ownership; SentiBio: Equity Ownership; Aptose: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Equity Ownership; United Therapeutics: Patents & Royalties: GD2 inhibition in breast cancer ; Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Astra Zeneca: Research Funding; Aptose: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Patents & Royalties: MDM2 inhibitor activity patent, Research Funding; Eutropics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Consultancy; Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. DiNardo:Abbvie: Honoraria; Karyopharm: Honoraria; Medimmune: Honoraria; Bayer: Honoraria; Celgene: Honoraria; Agios: Consultancy. Jain:Verastem: Research Funding; BMS: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Celgene: Research Funding; Adaptive Biotechnologioes: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Abbvie: Research Funding; Pharmacyclics: Research Funding; Astra Zeneca: Research Funding; Pharmacyclics: Research Funding; Abbvie: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Infinity: Research Funding; Incyte: Research Funding; Pfizer: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; ADC Therapeutics: Research Funding; Astra Zeneca: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Seattle Genetics: Research Funding; ADC Therapeutics: Research Funding; Infinity: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pemmaraju:Affymetrix: Research Funding; SagerStrong Foundation: Research Funding; plexxikon: Research Funding; daiichi sankyo: Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; abbvie: Research Funding; cellectis: Research Funding; stemline: Consultancy, Honoraria, Research Funding; novartis: Research Funding. Short:Takeda Oncology: Consultancy. Wierda:Genentech: Research Funding; AbbVie, Inc: Research Funding. Cortes:Novartis: Consultancy, Research Funding; Arog: Research Funding; Astellas Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding. Daver:Kiromic: Research Funding; BMS: Research Funding; Pfizer: Consultancy; Incyte: Consultancy; Karyopharm: Consultancy; Karyopharm: Research Funding; Incyte: Research Funding; Pfizer: Research Funding; ImmunoGen: Consultancy; Novartis: Research Funding; Novartis: Consultancy; Sunesis: Consultancy; Daiichi-Sankyo: Research Funding; Sunesis: Research Funding; ARIAD: Research Funding; Alexion: Consultancy; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal